Macrophages: the macrophage response following phagocytosis of apoptotic cells
Emeritus professor of TOHO UNIVERSITY.
|
Macrophages: the macrophage response following phagocytosis of apoptotic cells |
|
Kobayashi,Yoshiro, Emeritus professor of TOHO UNIVERSITY. |
![]() What is apoptosis?
What is apoptosis? ![]() Difference between apoptotic cells and live cells
Difference between apoptotic cells and live cells ![]() Our research (1)
Our research (1) ![]() Our research (2)
Our research (2) ![]() Our research (3)
Our research (3)
After publication of our paper in BBRC (239, 799-803, 1997), I decided to clarify the significance of this finding in vivo by examining (1) a response after whole body X-irradiation, (2) a response after injection of apoptotic cells into the peritoneal cavity and (3) Con A-induced hepatitis and later on (4) cytotoxic T cell induction after injection of apoptotic cells into the peritoneal cavity. Whole body X-irradiation at modest doses is known to induce apoptosis in the lymphoid tissues such as thymus, spleen and bone marrow, whereas Con A-induced hepatitis is known to be caused by acute apoptosis of hepatocytes upon activation of T cells and NK cells. Four years before, I also decided to examine the response following apoptosis of neutrophils infiltrated into female genital organs during estrous cycle.

In collaboration with the National Institute of Radiological Science, EU examined apoptosis of thymocytes and infiltration of neutrophils in association with whole body X-irradiation. As expected, he found the transient infiltration of neutrophils into thymus (J. Leukoc Biol 67, 780-84, 2000). The figure of histochemical analysis of neutrophil infiltration appeared on the front page, and the page is still hooked on the board outside of our laboratory.
After publication, another group in UK reported that whole body X-irradiation induces activation of splenic macrophages (Oncogene 20, 7085-95, 2001). Based on his contribution to science, EU received a Ph.D. degree in March, 1999.
Subsequently two TUs obtained interesting results on the role of neutrophils in regeneration of thymus, and then EN extended it by showing that neutrophils assist stromal cells with SDF-1 production (manuscript in preparation).
Subsequently TI obtained antibodies against MIP-2 and its receptor, CXCR2, and examined the effects on the response after injection of apoptotic cells into the peritoneal cavity. Then he examined the role of infiltrating neutrophils in the response after whole body X-irradiation.
When B10 mice were irradiated with 1 Gy of X-rays, neutrophil infiltration peaked at 12 hours, and it was significantly inhibited either by anti-MIP-2 antibodies or by anti-CXCR2 antibodies. In addition, when thymic macrophages were isolated from mice at the time of phagocytosis of apoptotic cells but not just after irradiation, they produced MIP-2 protein. These findings indicate that whole body X-irradiation is suitable for studying the response following phagocytosis of apoptotic cells in vivo.
When apoptotic cells and macrophages were stained with anti-ssDNA antibodies in brown and anti-F4/80 monoclonal antibody in blue, apoptotic cells inside macrophages were detected near the periphery of cortex in the panel iii but not other areas 9 hours after X-irradiation.
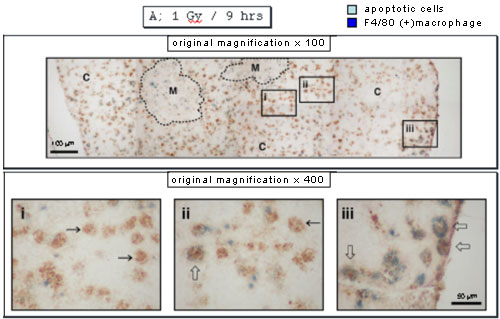
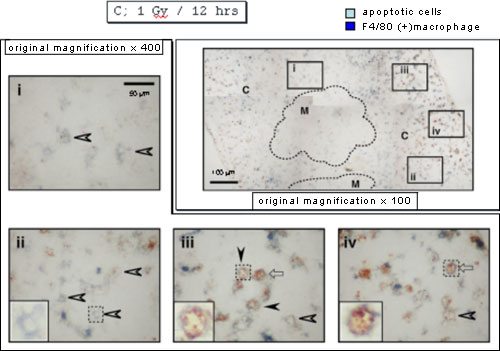
Twelve hours after X-irradiation, almost all the apoptotic cells disappeared, and there were macrophages with an empty inner area in the panel ii or containing a few apoptotic cells in the panels iii and iv, the former reflecting complete digestion of apoptotic cells and the latter incomplete digestion. Interestingly the former was detected mainly at the inner area of cortex near medulla.
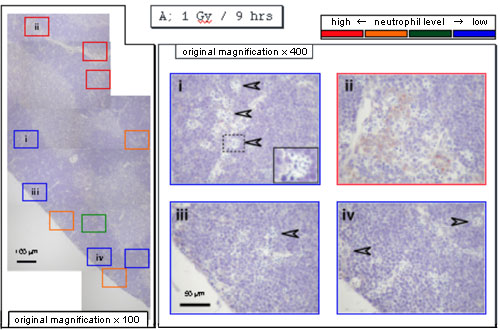
When neutrophils were stained with anti-myeloperoxidase antibodies, they were detected mainly near the vessels 9 hours after X-irradiation.
On the contrary, they were detected at the periphery of cortex 12 hours after X-irradiation, indicating that neutrophils move from vessels to the periphery. In agreement with the previous results, there were cells with an empty inner area in the panel iii or containing a few (apoptotic) cells in the panel ii.
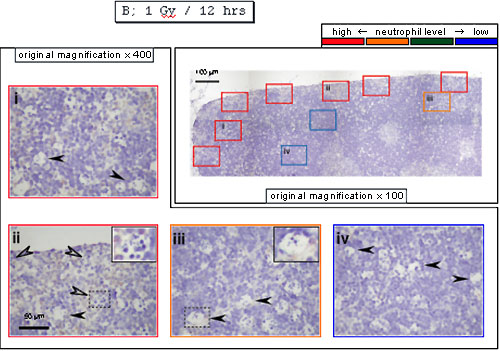
These results suggest that, as neutrophils move from vessels to the periphery, digestion of apoptotic cells in macrophages appears to be enhanced. In support of this, when neutrophil infiltration was suppressed with anti-MIP-2 antibodies, many cells appear to have a few (apoptotic) cells as shown in the panel ii even 12 hours after X-irradiation, indicating that digestion of apoptotic cells appear to be delayed. In addition, neutrophils enhanced digestion of apoptotic cells in macrophages in vitro (J. Immunol. 175, 3475-83, 2005). Based on his contribution to science, TI received a Ph. D. degree in March, 2005. Later, other researchers reported that Mincle recognizes dead cells in this model (Nat. Immunol. 9, 1179-1188, 2008).
“Cluster”-like structure as shown in ii in the above figure indicates phagocytosis of apoptotic cells. In such thymus, there are F4/80-positive macrophages, F4/80-negatve ones, infiltrating monocytes, monocytes-derived macrophages, and eosinophils. Which phagocytes are responsible for clearance of apoptotic thymocytes remains an unsolved important question.
Whole-body X-irradiation has been very useful for studying the response upon induction of acute and massive apoptosis in vivo. TS then decided to clarify the anti-inflammatory role of NO by using iNOS knockout mice in the model where late apoptotic cells were rarely detected (0.75 Gy).
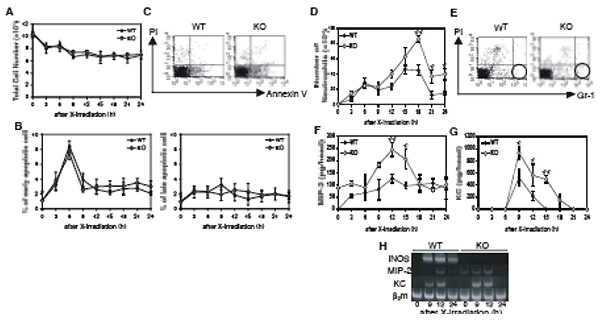
Although there was no difference in apoptosis between iNOS knockout mice and wild type mice (B), there were more neutrophils (D) and more MIP-2 (F, H) and KC (G, H) protein and mRNA in iNOS knockout mice than in wild type mice. The same was true when a NOS inhibitor, L-NAME, was administered to wild type mice. Under this condition, neither TGF-β mRNA nor IL-10 mRNA was detected in the thymus. Collectively these findings demonstrated that NO plays a critical role in preventing neutrophil infiltration upon induction of apoptosis. This was accepted as a cutting edge paper of J Immunol (J Immunol 179, 3407-3411, 2007). Although it was reported by administrating early apoptotic cells to the lung that TGF-β plays a similar role in the LPS-induced inflammation model in the lung (J Clin Invest 109, 41-50, 2002), it should be noted that his study shows the role of NO without administration of apoptotic cells under a much milder condition. TS was accepted as DC2 of the Japan Society for the Promotion of Science, probably because his publications in J Leukoc Biol and J. Immunol were highly appreciated, and received a Ph. D. degree in March, 2009.
Recently, HF examined the response after whole-body X-irradiation (4 Gy) by using IL-10 knockout mice. Although IL-10 mRNA was expressed in wild type mice under this condition, there were no changes in neutrophil infiltration and MIP-2 and KC production (BBRC 369, 432-436, 2008), suggesting the uniqueness of the inflammatory response induced by whole-body X-irradiation, which does not seem to be regulated by IL-10.
dichloromethylene bisphosphonate. When encapsulated into liposome and injected into mice, macrophages and immature dendritic cells take them and are killed by causing apoptosis.
RM examined neutrophil infiltration following injection of late apoptotic cells, which are otherwise called as secondary necrotic cells, into the peritoneal cavity of mice with sterile peritonitis induced by thioglycollate broth. He found the transient neutrophil infiltration and MIP-2 production. When macrophages were depleted by liposome containing clodronate*, both neutrophil infiltration and MIP-2 production were significantly suppressed (Apoptosis 6, 411-17, 2001).
CK and KK confirmed above results by using other late apoptotic cells and normal mice. Moreover when macrophages phagocytosing late apoptotic cells were isolated from the peritoneal cavity with a cell sorter, they were found to produce the comparable level of MIP-2 (BBA, 1541, 221-30, 2001).
Subsequently TI found that, following injection of late apoptotic cells, which are otherwise called as secondary necrotic cells, into the peritoneal cavity of mice with sterile peritonitis, anti-MIP-2 antibodies and anti-CXCR2 antibodies suppressed neutrophil infiltration to the level following injection of viable cells (Apoptosis 9, 485-93, 2004).
When NT examined neutrophil infiltration by injecting necrotic cells, which were obtained by freezing and thawing three times, into the peritoneal cavity, she found that KC was produced more significantly than by injecting late apoptotic cells and that anti KC antibody suppressed neutrophil infiltration to the similar extent as anti MIP-2 antibody (BBRC 361, 533-536, 2007). Whether or not necrotic cells and late apoptotic ones induce infiltration of different neutrophils awaits further study.
Con A-induced hepatitis is caused by activated T and NK cells-mediated apoptosis of hepatocytes, and has been widely investigated as a model of virus-induced hepatitis and autoimmune hepatitis. The other hepatitis model, endotoxin/galactosamine hepatitis, is caused by neutrophil-mediated necrosis of hepatocytes, which are triggered by TNF-α-induced apoptosis of hepatocytes (J Immunol 160, 3480-86, 1998). We then hypothesized that, in Con A-induced hepatitis, apoptosis of hepatocytes may induce neutrophil infiltration into liver, and that neutrophils may cause necrosis of hepatocytes.
TO found that Con A-induced hepatitis is greatly suppressed in mice rendered neutropenic with anti-Gr-1 monoclonal antibody. Subsequently SH found that IFN-γ production is also suppressed in these mice and that IFN-γ is mainly produced by T and NK cells in response to Con A with the help of neutrophils (Cell Immunol 233, 23-29, 2005).
Contrary to our expectation, neutrophils still infiltrated into the liver even when apoptosis was inhibited by a caspase inhibitor, Z-VAD-fmk. Furthermore, when MH examined the effects of Kupffer cell depletion on Con A hepatitis, apoptosis of hepatocytes, MIP-2 and KC production and neutrophil infiltration were significantly suppressed (Cell Immunol in press). Therefore, contrary to our initial hypothesis, it is likely that Con A-activated T and NK cells may produce MIP-2 and KC, which is dependent on Kupffer cells, and we are considering that Con A hepatitis is rather inadequate for studying the response upon induction of massive apoptosis.
YS detected allo-killer T cell activity in the peritoneal cavity after injection of apoptotic P388 cells, which are derived from DBA/2 mice, into the peritoneal cavity of C57BL/6 mice. She then examined the role of neutrophils in cytotoxic T cell induction. Neutrophil depletion by anti Gr-1 mAb and suppression of neutrophil infiltration by anti MIP-2 antibody caused suppression of MCP-1 production and cytotoxic T cell infiltration (J Leukoc Biol 81, 412-420, 2007). She also found that MCP-1 is not produced by infiltrating neutrophils but that neutrophils assist other cells with MCP-1 production (unpublished). Based on her contribution to science, YS received a Ph. D. degree in March, 2007.
MT then examined what type of cells transport P388 cell antigens from peritoneal cavity to parathymic lymph nodes and then to spleen upon injection of X-irradiated P388 cells. He found that infiltrating neutrophils and monocytes butnot immature dendritic cells transport them (BBRC 377, 589-594, 2008). This is the first to show migration of infiltrating neutrophils into draining lymph nodes after phagocytosing tumor cells, although induction of specific killer T cell has not been proved. Based on his contribution to science, MT received a Ph. D. degree in March, 2008.
SS undertook a series of experiments to clarify whether neutrophils infiltrating into female genital organs during estrous cycle undergo apoptosis, whether such neutrophils are phagocytosed by macrophages, and what is the following response.
It is known that neutrophils are accumulated into the female genital organs through MIP-2, and that neutrophils are exhausted at estrus. Because it is expected that such neutrophils should keep vagina pathogen-free, he examined the effect of neutrophil deletion by ant Gr-1 mAb. Although Gr-1 antigen is also weakly expressed on eosinophils, he found that neutrophils but not eosinphils are depleted from the female genital organs. As expected, the number of cocci was increased upon neutrophil depletion. Part of neutrophils remained alive, suggesting that they may play a role in addition to killing bacteria. To our surprise, neutrophil depletion caused a blockading of estrous cycle at diestrus and affected the levels of estrogen and progesterone (BBRC 382, 35-40, 2009). There is evidence that neutrophils regulate the estrous cycle through opioid peptide release (under submission). Based on his contribution to science, SS received a Ph. D. degree in April, 2009.
![]()
BACK / HOME(English) / NEXT