Macrophages: the macrophage response following phagocytosis of apoptotic cells
Emeritus professor of TOHO UNIVERSITY.
|
Macrophages: the macrophage response following phagocytosis of apoptotic cells |
|
Kobayashi,Yoshiro, Emeritus professor of TOHO UNIVERSITY. |
![]() What is apoptosis?
What is apoptosis? ![]() Difference between apoptotic cells and live cells
Difference between apoptotic cells and live cells ![]() Our research (1)
Our research (1) ![]() Our research (2)
Our research (2) ![]() Our research (3)
Our research (3)
In the fall of 1995, many undergraduate students visited my laboratory to hear what is going on here with hope to join and pursue their work for thesis. At that time I was interested in cytokine production by macrophages phagocytosing apoptotic cells, partly because to my knowledge nobody has published such work.
There was one report that thromboxane B2, one of a marker of inflammation, is not produced by macrophages upon coculturing with apoptotic cells (J. Leukoc. Biol. 52, 269-73, 1992). Based on this finding, J. Savill and his colleagues suggested in 1993 that the macrophage response is nonphlogistic upon phagocytosis of apoptotic cells (Immunol Today 14, 131-36, 1993). But they had not examined cytokine production by macrophages upon coculturing with apoptotic cells. They then published the result that GM-CSF is not produced by the macrophages (Am J Pathol 149, 911-21, 1996) in 1996, one year after we planned following experiments.
Of course we expected that macrophages produce anti-inflammatory cytokines such as IL-10, TGF-β and IL-1 receptor antagonist upon coculturing with apoptotic cells. But just for my curiosity and to control our experiments, we also planned to examine proinflammatory cytokines, such as IL-1α, IL-1β and TNFα. I decided that the theme is assigned to EU (a graduate student at the 1st year of the doctor course) and TK (an undergraduate student).
Next year (1996), their work started. They obtained apoptotic CTLL-2 cells by culturing in IL-2-free medium for 28 hours (late apoptotic cells, which are otherwise called as secondary necrotic cells) and cocultured them with thioglycollate broth-induced mouse peritoneal macrophages.
Their results were quite unexpected, because coculturing of macrophages with apoptotic cells induced production of a significant amount of MIP-2 mRNA and because peritoneal injection of the culture supernatant induced a significant infiltration of neutrophils into the cavity. The latter finding had been obtained by DY (a then post doctoral fellow).
To our embarrassment, we initially planned to examine IL-8 but not MIP-2, because I did not know that murine homologue of IL-8 is called MIP-2. That was so unexpected.
All the results were finally obtained one year later. I tried to publish them in a so-called big journal in vain. It was finally accepted in BBRC (BBRC 239, 799-803, 1997) and published in October 29, 1997.
To our surprise, in the next month, R.E. Voll et al. reported a letter entitled "Immunosuppressive effects of apoptotic cells" in Nature that coculturing of human monocytes with early apoptotic cells, which are otherwise called as apoptotic cells, induces production of IL-10 (Nature, 390, 350-51, 1997). Since it is known that peripheral blood human monocytes cannot phagocytose apoptotic cells (Nature 242, 170-73, 1990), the phenomena are not related to phagocytosis. Recently, however, we found that phagocytosis is not mandatory for cytokine production during cococulturing macrophages with apoptotic cells.
Subsequently Fadok et al. reported that coculturing of human monocyte-derived macrophages with early apoptotic cells, which are otherwise called as apoptotic cells, induces production of TGF-β but not IL-10, IL-8 and IL-1β in Feburuary 15, 1998 (J Clin Invest 101, 890-98, 1998).
TGF-β is secreted as an inactive precursor, which is then subject to limited proteolysis for activation. Commercially available ELISA kits are said to be unable to discriminate a precursor form of TGF-β from a mature form of TGF-β, and consequently researchers measure the level of TGF-β after treatment of samples with acid ethanol to convert all TGF-β to a mature form. Since it is known that serum contain a significant amount of TGF-β, researchers including Fadok have conducted experiments under a serum-free condition. On the other hand, serum and their components are reported to enhance phagocytosis of apoptotic cells, and consequently a serum-free condition might not reflect physiological situations.
These two papers, in particular the paper of Fadok, have been very frequently cited by other researchers, probably because their results appear to explain that apoptotic cells are cleared up without causing inflammation in vivo.
In 1996, another undergraduate student, KK, engaged in the experiments for her thesis to quantitate phagocytosis of apoptotic cells by macrophages. In 1997, KK entered our graduate school, and continued her work on quantitation of phagocytosis of apoptotic cells by macrophages with a flow cytometer and semi-quantitation of cytokine mRNA upon coculturing of PMA-treated THP-1 cells (human macrophages) with apoptotic CTLL-2 cells. She obtained the results showing that, as apoptosis proceeds from an early stage to a late stage, apoptotic cells are phagocytosed more significantly and a more significant amount of IL-8 is produced upon coculturing of apoptotic cells with macrophages. Her work was submitted to J, Immunol in February, 1998, accepted in July and published in December (J. Immunol. 161, 6245-49, 1998). KK was then accepted as DC1 of the Japan Society for the Promotion of Science, probably because her publication in J.Immunol was highly appreciated.
Researchers in this field, including our group, had used human monocyte-derived macrophages, PMA-treated THP-1 cells or thioglycollate broth-induced peritoneal macrophages but not resident tissue macrophages.
KK then decided to examine the responses of Kupffer cells and alveolar macrophages upon coculturing with apoptotic cells, and found the essentially same results as PMA-treated THP-1 cells, human monocyte-derived macrophages and thioglycollate broth-induced peritoneal macrophages that MIP-2 is produced upon coculturing of macrophages with late apoptotic cells, which are otherwise called as secondary necrotic cells. In addition, upon coculturing with early apoptotic cells, which are otherwise called as apoptotic cells, a lesser amount of MIP-2 was also produced. Her work clearly demonstrated that MIP-2 is produced upon coculturing of even normal tissue macrophages with apoptotic cells. Although her work was initially submitted to J. Leukoc. Biol., reviewers criticized very regrettably that it just confirmed previous findings. Although it was finally accepted to another journal (Cell Immunol 211, 1-7, 2001), I still believe that this work is very important in this field.
Recently TM found that alveolar macrophages phagocytose apoptotic cells less efficiently than peritoneal macrophages, whereas alveolar macrophages produce more MIP-2 than peritoneal macrophages upon coculturing with apoptotic cells (unpublished results), suggesting that phagocytosis is not always necessary for MIP-2 production. In support of this, cytochalasin B inhibited phagocytosis almost completely but not MIP-2 production. TY then extended these observations by showing that, upon coculturing with late apoptotic cells, peritoneal macrophages and M-CSF-induced bone marrow macrophages produced IL-10 and MIP-2, which was similar to M2 type of macrophages, whereas alveolar macrophages and GM-CSF-induced bone marrow macrophages produced IL-12p40 and MIP-2 which was similar to M1 type of macrophages (Cell Immunol 251, 124-130, 2008). Such dichotomy was confirmed by other researchers. Based on these contributions to science, TY received a Ph. D. degree in March, 2009.
As described above, KK also conducted experiments with human monocyte-derived macrophages upon a reviewer' request when her first paper was submitted to J. Immunol, and she obtained the similar results as PMA-treated THP-1 cells. It was expected but very important, because we used M-CSF but not human serum to differentiate human monocytes to macrophages. Of note is that Fadok and many other researchers have used human serum for that purpose.
This is because I had met a difficulty in obtaining monocyte-derived macrophages with human serum reproducibly.
KK decided to examine the effect of human serum on the response of human macrophages, the results being quite astonishing. The addition of human serum to a coculture of PMA-treated THP-1 cells with late apoptotic CTLL-2 cells led to a suppression of IL-8 production. The same was true with human monocyte-derived macrophages and other apoptotic cells at either early or late stages, but necrotic cells, which were obtained by three times freeze-thawing, did not cause such suppression.
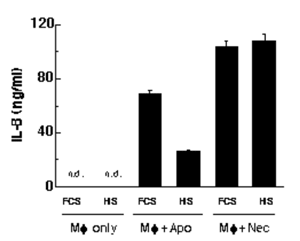
When human IgG was added to the coculture instead of human serum, IL-8 production was also suppressed to a lesser extent. Either human serum or human IgG induced IL-10 and TGF-β production. Since human IgG binds to FcγRI with a high affinity, she then examined the effect of down modulation of FcγRI with monoclonal antibody (M22). As shown in the figure below (black bars), such down modulation totally abolished the effects of human serum and human IgG.
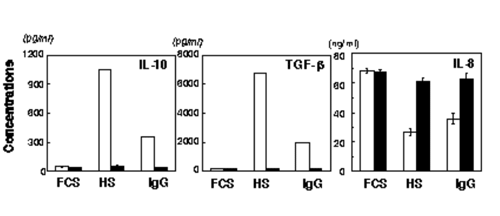
When KK presented her data at our seminar for the first time, the data were so beautiful that I could not believe in them instantly. Since the effect of human serum was more significant than that of human IgG, the possibility remains that other proteins may also bind to FcγRI.
Because she found by chance that commercially available ELISA for TGF-β (Biosource) can measure a mature form of TGF-β specifically even in the presence of serum, she has not treated the samples with acid ethanol before measurement.
These results can explain well why we and Fadok'group found the inflammatory and anti-inflammatory responses upon coculturing of macrophages with apoptotic cells. Moreover, these results also suggest that, when apoptosis occurs in the tissue, neutrophils infiltrate for unknown reasons and that, when serum components permeate into the tissue, such an inflammatory response is then suppressed.
When I told this idea to a famous immunologist, she answered that serum components are always present in the tissue, otherwise the cells in the tissue must not be alive. However it is well known that inflammation causes a significant increase in permeability of blood vessels as compared with normal vessels. We are considering that minute amounts of serum components may be present in the normal tissue.
She had a difficulty in publishing the results, but two years later it was accepted to the journal (J. Leukoc Biol 71, 950-6, 2002). Then KK published her hypothesis for the regulatory mechanism of macrophage response following phagocytosis of apoptotic cells as a minireview in SEIKAGAKU (75, 59-62, 2003).
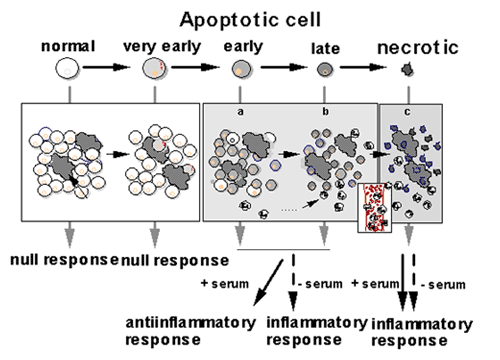
(SEIKAGAKU 75, 59-62, 2003)
As described previously, when apoptosis proceeds from an early stage to a late stage, apoptotic cells are cleared up more significantly and a more significant amount of IL-8 is produced upon coculturing with macrophages. Then KK decided to examine the response of macrophages upon coculturing with very early apoptotic cells which express only a very small amount of PS.
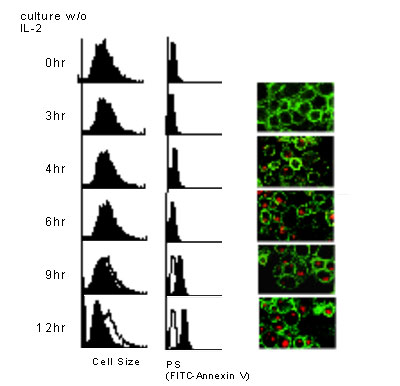
As shown in the figure above, a small number of very early apoptotic cells, obtained by culturing in IL-2-free medium for only 4 hours, were phagocytosed by macrophages. Such apoptotic cells induced production of a very small amount of IL-8, IL-10 or TGF-β, the response being almost null (J. Immunol. 171, 4672-79, 2003). The hypothesis presented previously includes these findings.
Apoptotic cells are very hard to be detected in normal body, and this is because apoptotic cells are cleared up as soon as they appear in vivo. In support of this notion, apoptotic cells are detected inside of macrophages in the normal thymus (Nature 372, 100-103, 1994). Consequently KK’s findings are quite relevant from the physiological point of views. Based on these contributions to science, KK received a Ph. D. degree in March, 2002.
Unexpectedly, SS found that early apoptotic cells are not swiftly phagocytosed by macrophages as compared with late apoptotic cells (J Biochem 141, 301-307, 2007). Since it is well known that apoptosis proceeds more rapidly and not synchronously at a single cell level, it is possible that a small number of late apoptotic cells, which are otherwise called as secondary necrotic cells, exist even in a population rich in early apoptotic cells, and that the number of late apoptotic cells increases in a time dependent manner.
Adiponectin is an important molecule linking obesity with insulin resistance and heart failure, and its plasma level decreases upon obesity. Since adiponectin is reported to suppress the production of inflammatory cytokines and enhance the production of anti-inflammatory cytokines upon LPS stimulation of macrophages, it has been considered anti-inflammatory hormone. On the contrary, SS found that high molecular weight adiponection from human plasma suppresses phagocytosis of late apoptotic cells by monocyte-derived macrophages and IL-8 production in the absence of LPS, but that it enhances both phagocytosis and IL-8 production in the presence of LPS, in collaboration with Dr. Nakano of Showa University. Consequently, adiponectin is not anti-inflammatory hormone, but rather a dual modulator of innate immunity. This finding also provides another regulatory mechanism of the macrophage response following phagocytosis of apoptotic cells (BBRC 334, 1180-83, 2005).
Based on these contributions to science, SS received a Ph. D. degree in March, 2006.
In summary, a series of our study showed that macrophages produce IL-8 or MIP-2 but not TNF-a or IL-1 upon coculturing with late apoptotic cells, which are otherwise called as secondary necrotic cells, and identified several mechanisms to prevent such a response, including (1) suppression by human serum and human IgG, (2) an almost null response upon coculturing with very early apoptotic cells and (3) suppression by adiponectin. However the suppressive effect of serum and normal IgG in mice has not been so strong as that in humans.

Recently TS found that macrophages produce a large amount of nitric oxide (NO) upon coculturing with early apoptotic cells and that NO then suppress MIP-2 production by macrophages (J Leukoc Biol 80, 744-752, 2006). Interestingly, macrophages did not produce NO upon coculturing with late apoptotic cells that are otherwise called as secondary necrotic cells. Exogenously added NO donor inhibited ERK phosphorylation and IkB degradation in macrophages upon coculturing with late apoptotic cells, and thereby suppressed MIP-2 production. Moreover, although TGF-β was produced upon coculturing of macrophages with early apoptotic cells, anti-TGF-β antibody did not affect MIP-2 production. Collectively his findings clarified the mechanism for (2) described above, and pointed to the importance of NO in regulating MIP-2 production. His paper was selected as a Pivotal Advance of J Leukoc Biol for the first time as a Japanese researcher. I was phone-interviewed by Dr. Oppenheim, an Editor, and my portrait appeared on the front page, which some may have known through our university’s monthly news (Toho Now).
![]()
BACK / HOME(English) / NEXT