Macrophages: the macrophage response following phagocytosis of apoptotic cells
Emeritus professor of TOHO UNIVERSITY.
|
Macrophages: the macrophage response following phagocytosis of apoptotic cells |
|
Kobayashi,Yoshiro, Emeritus professor of TOHO UNIVERSITY. |
![]() What is apoptosis?
What is apoptosis? ![]() Difference between apoptotic cells and live cells
Difference between apoptotic cells and live cells ![]() Our research
Our research
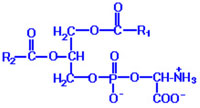
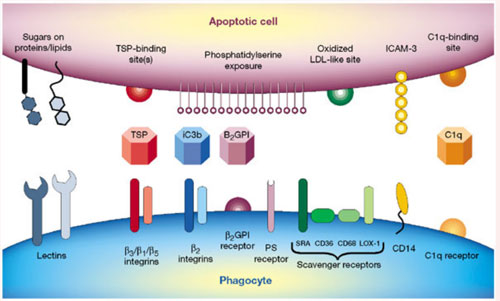
Apoptotic cells are discriminated from live cells and cleared up by phagocytes such as macrophages. Then what is the significant difference between apoptotic cells and live ones? It is the amount of phosphatidylserine (PS) on the cell surface. The structure of it is shown left, where R1 and R2 indicate alkyl groups. One is localized in the inner side of cell membrane of live cells, whereas it is externalized in apoptotic cells. Such externalized PS molecules are recognized by a variety of surface molecules including SR-A, CD14 and CD36, Tim4, and BAI1.
The other is altered sugar chains. When apoptosis occurs, glycoproteins with particular types of sugar chains are distributed on the cell membrane in a cluster-like fashion and are recognized by lectin-like molecules, such as necleolin. Alternatively, apoptotic cells and phagocytes are bridged by thrombospondin, which is recognized by unknown molecules on apoptotic cells, and αvβ3 on phagocytes, or by MFG-E8, which is recognized by PS on apoptotic cells, and αvβ3 on phagocytes.
(Nature 407, 784-88, 2000)
TSP:thrombospondin
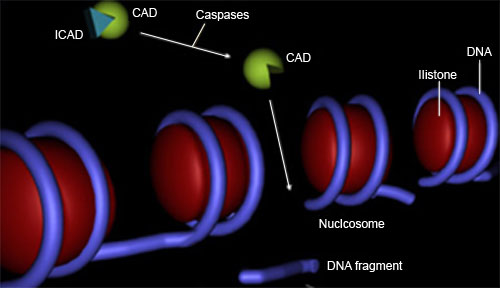
They belong to a group of cysteine proteases, and cleave substrates at the carboxy end of aspartic acid. There are initiator caspases such as caspase 8 and effector caspases such as caspase 3, the former activating the latter with limited proteolysis
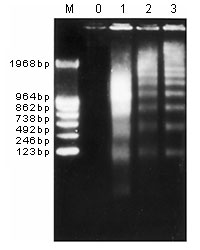
DNA winds around histone to form nucleosome. Apoptosis induces cleavage of naked DNA between nucleosomes with caspase-activated DNase (CAD), which produces ladder upon agarose electrophoresis.
Even in some necrotic cells, PS is externalized. In other words, PS externalization does not guarantee apoptosis.
After apoptosis starts, PS is externalized in association with a decrease in cell size, caspase* activation and DNA degradation (ladder formation*). The cells are then stained with dyes such as propidium iodide (PI), but do not leak cytosolic proteins such as lactate dehydrogenase. Later the cells leak cytosolic proteins.
Externalized PS on the cell surface is often detected with fluorochrome-labeled annexin V by a flow cytometer. Here annexin V-positive but PI-negative cells are defined as early apoptotic cells, whereas annexin V-positive and PI-positive cells are defined as late apoptotic cells. When cytosolic proteins are leaked from cells, such cells were named secondary necrotic cells (see below). Of note is that late apoptotic cells do not always leak cytosolic proteins.
For instance, when CTLL-2 cells, an IL-2-dependent cell line, are cultured in the absence of IL-2, they undergo apoptosis. The time kinetics of a decrease in cell size, PS externalization and caspase activation are shown below. Nine hours after culture, cells began to be stained with annexin V and caspase activity in the cells began to rise. Even 12 hours after culture, cells are not stained with PI.
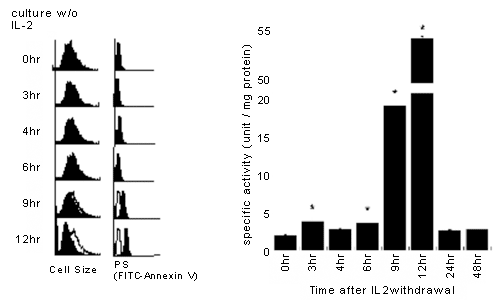
Some researchers call annexin-V-positive but PI-negative cells as apoptotic cells, whereas they call annexin V-positive and PI-positive cells as secondary necrotic cells.
For instance, the researchers who try to differentiate apoptotic cells from necrotic cells in tissue sections have defined these two types of death in a following way.
We followed simple yet very useful criteria to define necrosis and apoptosis. Necrosis is defined by the uptake of PI (indicating damage to the cell membrane) and the lack of nuclear condensation and fragmentation. Apoptosis is defined by the lack of PI uptake (indicating the integrity of the cell membrane) and the presence of clear nuclear condensation and/or fragmentation. Secondary necrosis is defined by the presence of nuclear condensation and/or fragmentation along with PI uptake. These are (late) apoptotic cells (and are counted as such) that sustained some terminal damage to the cell membrane before they were cleared. (Am J Physiol Cell Physiol 284, C1309-C1318, 2003)
According to their definition, secondary necrosis is a synonym of late apoptosis (see the underlined sentences).
Other researchers have proposed the different definition as follows.
Beyond 18 h in culture, a steadily increasing proportion of senescent neutrophils exhibit a characteristic late apoptotic morphology in which nuclear degradation or so-called evanescence is accompanied by electron microscopic evidence of limited granule fusion, we propose that neutrophils with classical features of apoptosis should be regarded as early apoptotic cells.(J. Immunol. 166, 4743-4750, 2001)
According to their definition and rationale, apoptosis should be called early apoptosis (see the underlined sentence).
Consequently, we note, whenever possible, that early apoptosis is otherwise called apoptosis, whereas late apoptosis is otherwise called secondary necrosis, hopefully diminishing confusion.
Of note is that apoptotic cells are very hard to be detected in normal body. This is because apoptotic cells are cleared up as soon as they appear in vivo. For instance, there is a report that apoptotic cells are detected only inside macrophages in the normal thymus with a TUNEL method* (Nature 372, 100-03, 1994).
One might wonder if apoptotic cells are engulfed as soon as they appear. When SS examined this under a time-lapse fluorescence microscope, he found that the attachment of early apoptotic cells to macrophages does not cause rapid phagocytosis, as compared with late apoptotic cells (J Biochem 141, 301-307, 2007).
Now we are ready for introducing our recent study.
![]()
BACK / HOME(English) / NEXT